Written by: Justin Seltzer, MD (NUEM PGY-3) Edited by: Priyanka Sista, MD, (NUEM PGY-4) Expert commentary by: Peter Pruitt, MD, MS
Make sure to check out The ED Guide to Neuroimaging: Part 1
Part two of this series examines the literature regarding the appropriate use of the head CT in blunt head trauma, a common clinical grey zone in emergency medicine.
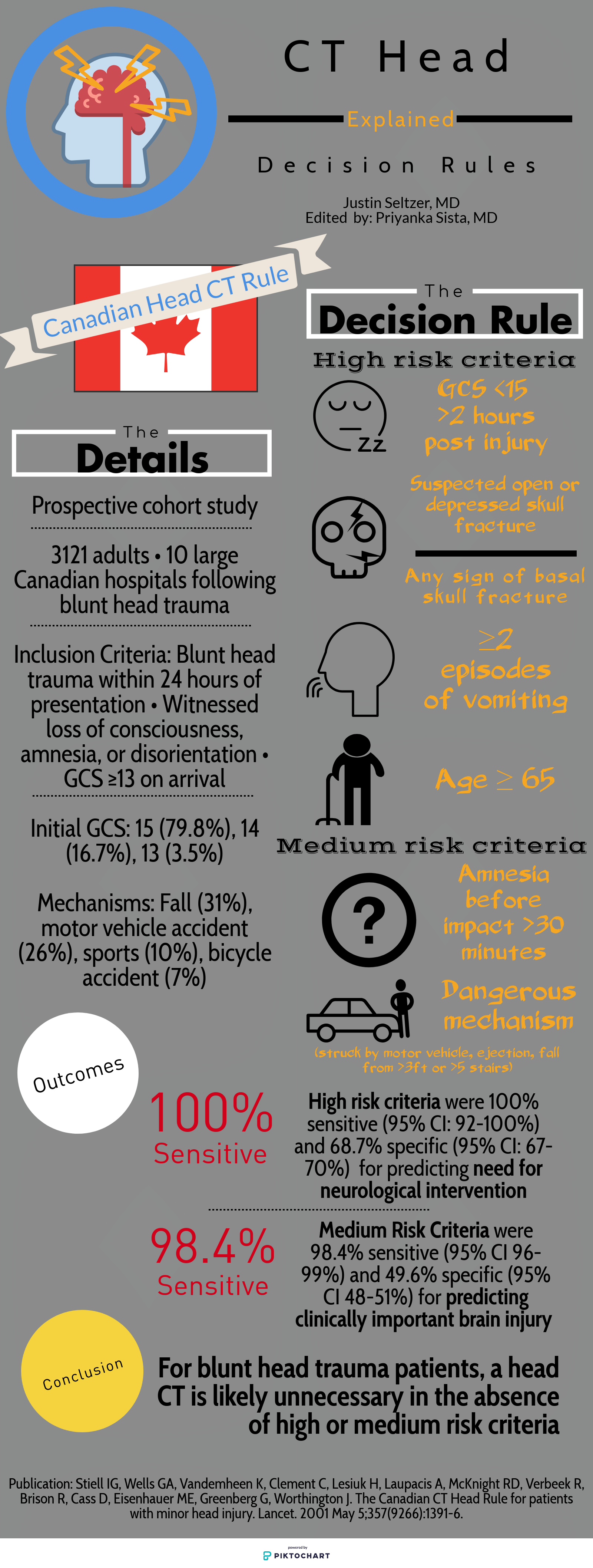
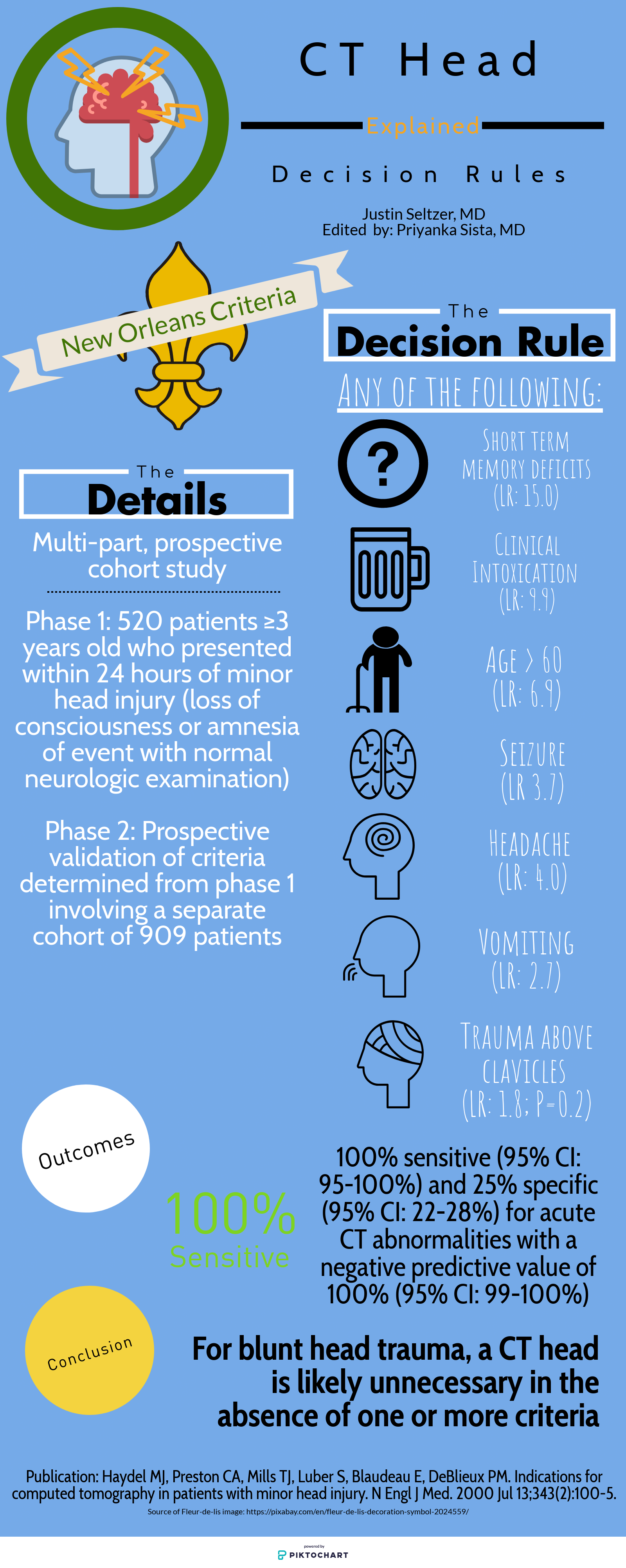
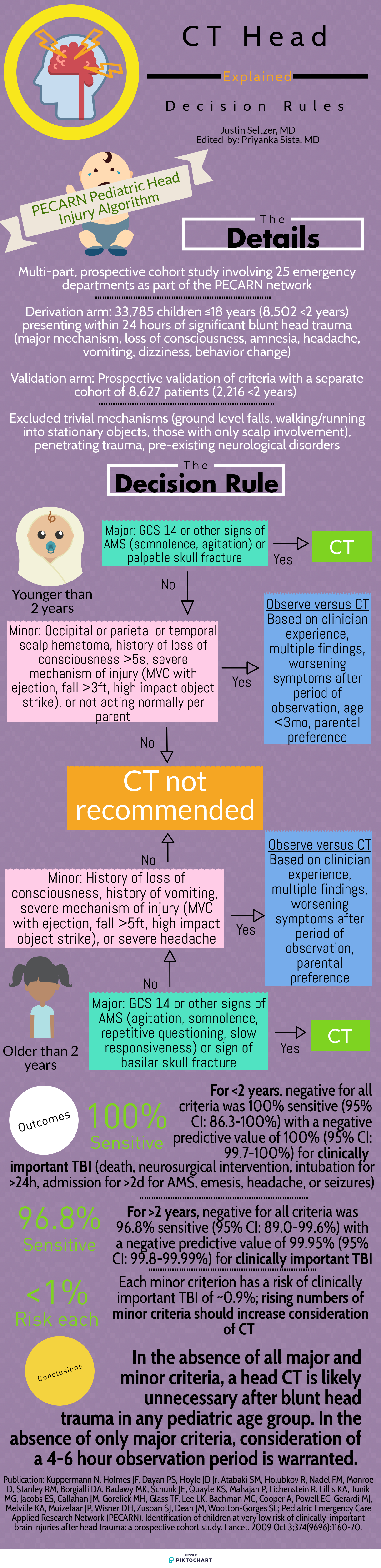
The Canadian Head CT Rule (Canadian), New Orleans Criteria (New Orleans), NEXUS II Head CT Rule (NEXUS), and PECARN Pediatric Head Injury Algorithm (PECARN) are four major decision rules designed to assist clinicians with this often difficult decision. This article is dedicated to comparing these rules and providing a reasonable guide for maximizing their individual utility. The provided infographics detail the specifics of each rule for quick reference.
To start, there are many shared characteristics between the rules. All apply to blunt head trauma only and, except for NEXUS, specifically to those presenting within 24 hours of injury. They utilize criteria to characterize high risk populations for which emergent head CT is appropriate as well as those low risk enough to forego it. Each boasts near perfect sensitivity and negative predictive values for clinically significant acute intracranial processes. Finally, all were prospective cohort studies and, aside from New Orleans, multi-center.
However, there are differences between each rule that can impact their applicability to certain situations and populations.
Study population: The single center New Orleans Criteria had the smallest study population, with 1429 total patients, while the largest, PECARN, had over 42,000 patients. All aside from NEXUS had some age restrictions. Canadian included adults and pediatric patients older than 16 years and New Orleans included adults and pediatric patients older than 3 years. PECARN was exclusively pediatric and excluded anyone over 18 years old.
Inclusion and exclusion criteria: There was significant heterogeneity between the studies on what qualified for inclusion. New Orleans only included patients with known loss of consciousness or post-injury amnesia with a normal neurologic exam. Similarly, Canadian involved patients with GCS ≥13 and witnessed alteration or loss of consciousness. PECARN, on the other hand, was most concerned with mechanism and excluded patients with trivial mechanisms or injuries, such as ground level falls, walking into objects, and isolated scalp involvement. These are further contrasted with NEXUS, which included “all patients with blunt trauma with minor head injury (Glasgow Coma Scale [GCS] score of 15) who present to participating study center.”[1]
Decision rule criteria: Certain criteria, such as evidence of skull fracture, persistent vomiting, older age (>60-65 years), were nearly universally present. However, beyond these there is little consensus. NEXUS, likely because it applies to all ages, includes criteria such as alertness, behavior changes, and scalp hematoma similar to PECARN. Only New Orleans included clinical intoxication, while NEXUS was the only rule to include coagulopathy. Mechanism-based criteria were only considered by Canadian and PECARN.
Primary outcome: There is significant similarity in terms of primary outcome. NEXUS criteria sought “clinically important intracranial injury,” New Orleans any acute abnormality on CT, and PECARN “clinically important traumatic brain injury.” The definitions varied somewhat but were generally similar. Only Canadian stratified differently, with a set of criteria geared towards identifying the need for neurosurgical intervention specifically and another set for the more familiar “clinically important brain injury.”
Methods of Application: NEXUS, Canadian, and New Orleans are all or nothing; meeting even one element results in a head CT and not meeting any means a head CT is likely unnecessary. PECARN is unique in that if the major criteria are not met, minor criteria defer to observation or head CT based in part on non-standardized elements such as physician experience and parental preference. Only in the absence of major and minor criteria can a child be cleared immediately.
Finally, it is important to understand the level of external validation and comparison to which each of these studies has been subjected. Boudia and colleagues performed an external validation study of both Canadian and New Orleans involving 1582 patients 10 years and older over a 3-year period. They noted some key differences between reported performance and performance between the two metrics. Canadian had 100% sensitivity for need for neurosurgical intervention, while New Orleans was 82% sensitive. Canadian was 95% sensitive for clinically significant head CT findings, compared with 86% sensitivity for New Orleans. Negative predictive values were 100% and 99% for Canadian and New Orleans, respectively.[5] Mower, Gupta, Rodriguez, and Hendey recently published a nearly 10 year validation of NEXUS involving 11,770 patients from four centers, which showed improved sensitivity (99% versus 98.3%), specificity (25.6% versus 13.7%), and negative predictive value (99.7% versus 99.1%) compared with the original study for clinically significant intracranial injury. This study also compared NEXUS and Canadian performance within the same study population for those who met Canadian criteria. NEXUS was found to have superior sensitivity (100% versus 97.3%) but worse specificity (32.6% versus 58.8%) for neurosurgical intervention while having worse sensitivity and specificity (97.7%/12.3% versus 98.4%/33.3%) compared with Canadian medium risk criteria for identification of significant brain injury.[6] Schachar and colleagues compared New Orleans, Canadian, and NEXUS in 2,101 pediatric patients over nearly seven years at a non-trauma center; all showed negative predictive values over 97% however Canadian and NEXUS both showed dramatically lower (65.2% and 78.3%, respectively) sensitivities in this population.[7] Smits and colleagues concluded from a Dutch cohort of 3,181 adult patients that Canadian had a lower sensitivity than New Orleans for traumatic intracranial findings but still identified all neurosurgical cases and had a much higher specificity, resulting in a greater number of avoided unnecessary scans.[8] In contrast, PECARN has been externally validated multiple times, all with near perfect sensitivity and negative predictive value;[9-13] of note, in one study two physically abused children with clinically important traumatic brain injury were misclassified as low risk, highlighting a gap in its criteria.[11]
In summary, the four major head CT decision rules all boast impressive sensitivity and negative predictive value for significant traumatic intracranial injury, though external validation and comparison studies have shown that some rules perform better than others under less controlled conditions. When properly applied to the intended patient populations, we can conclude that these are all useful clinical decision making tools, in particular to identify low risk patients and avoid unnecessary radiation exposure, costs, and resource utilization.
Expert Commentary
I applaud Dr. Seltzer for his interesting and informative summary of decision instruments for patients with blunt head trauma. It is important to have a clear strategy for managing patients with this complaint, since traumatic brain injury is one of the most common ED complaints, accounting for an estimated 2.8 million annual visits in 2013, and the number of visits are steadily increasing.[1] Using a well validated decision instrument, such as the Canadian CT Head Rule in adults or the PECARN rule in children, reduces the frequency of unnecessary imaging and decreases length of stay while increasing the diagnostic yield (frequency of positive tests) amongst those patients that are imaged.[2,3] With this in mind, integration of these rules into clinical practice is a key component of appropriate resource utilization, and is recommended by multiple clinical practice guidelines.[4–6] However, the use of decision instruments cannot completely replace clinical gestalt, defined as the impression of the patient derived from the clinical evaluation. Unfortunately, studies comparing decision instruments to gestalt are extremely limited.[7] One study compared the PECARN decision instrument to clinician gestalt and found gestalt to be much more specific with similar sensitivity, although clinicians were asked about the criteria used in the decision instruments prior to making their “gestalt” decision.[8] There are no studies comparing the decision instruments used in adults to gestalt, so their relative performance is still open to assessment. Clinical instinct is still a valuable tool, and decision instruments only function to support this core skill. It is also important to consider what constitutes a positive outcome in these studies. Most notably, the Canadian CT Head Rule in its simplest form does not attempt to identify individuals who will have no hemorrhage at all.[9] Instead, the authors pre-defined defines a “clinically important injury”, which allowed patients to have small subdural hematomas or trace subarachnoid hemorrhage while still being considered low risk by the rule. Because these lesions rarely require intervention, the clinical significance of identifying them is minimal.
Peter Pruitt, MD, MS
Assistant Professor
Department of Emergency Medicine
Northwestern University
How to cite this post
[Peer-Reviewed, Web Publication] Seltzer J, Sista P, (2019, December 15 ). The ED Guide to Neuroimaging: Part 2. [NUEM Blog. Expert Commentary by Pruitt P ]. Retrieved from http://www.nuemblog.com/blog/emergency-neuroimaging-pt2.
Other posts you might enjoy…
References
Mower WR, Hoffman JR, Herbert M, Wolfson AB, Pollack CV Jr, Zucker MI; NEXUS II Investigators. Developing a decision instrument to guide computed tomographic imaging of blunt head injury patients. J Trauma. 2005 Oct;59(4):954-9.
Kuppermann N, Holmes JF, Dayan PS, Hoyle JD Jr, Atabaki SM, Holubkov R, Nadel FM, Monroe D, Stanley RM, Borgialli DA, Badawy MK, Schunk JE, Quayle KS, Mahajan P, Lichenstein R, Lillis KA, Tunik MG, Jacobs ES, Callahan JM, Gorelick MH, Glass TF, Lee LK, Bachman MC, Cooper A, Powell EC, Gerardi MJ, Melville KA, Muizelaar JP, Wisner DH, Zuspan SJ, Dean JM, Wootton-Gorges SL; Pediatric Emergency Care Applied Research Network (PECARN). Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009 Oct 3;374(9696):1160-70.
Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000 Jul 13;343(2):100-5.
Stiell IG, Wells GA, Vandemheen K, Clement C, Lesiuk H, Laupacis A, McKnight RD, Verbeek R, Brison R, Cass D, Eisenhauer ME, Greenberg G, Worthington J. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001 May 5;357(9266):1391-6.
Bouida W, Marghli S, Souissi S, Ksibi H, Methammem M, Haguiga H, Khedher S, Boubaker H, Beltaief K, Grissa MH, Trimech MN, Kerkeni W, Chebili N, Halila I, Rejeb I, Boukef R, Rekik N, Bouhaja B, Letaief M, Nouira S. Prediction value of the Canadian CT head rule and New Orleans for positive head CT scan and acute neurosurgical procedures in minor head trauma: a multicenter external validation study. Ann Emerg Med. 2013 May;61(5):521-7.
Mower WR, Gupta M, Rodriguez R, Hendey GW. Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) Head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study. PLoS Med. 2017 Jul 11;14(7):e1002313.
Schachar JL, Zampolin RL, Miller TS, Farinhas JM, Freeman K, Taragin BH. External validation of New Orleans (NOC), the Canadian CT Head Rule (CCHR) and the National Emergency X-Radiography Utilization Study II (NEXUS II) for CT scanning in pediatric patients with minor head injury in a non-trauma center. Pediatr Radiol. 2011 Aug;41(8):971-9.
Smits M, Dippel DW, de Haan GG, Dekker HM, Vos PE, Kool DR, Nederkoorn PJ, Hofman PA, Twijnstra A, Tanghe HL, Hunink MG. External validation of the Canadian CT Head Rule and New Orleans for CT scanning in patients with minor head injury. JAMA. 2005 Sep 28;294(12):1519-25.
Schonfeld D, Bressan S, Da Dalt L, Henien MN, Winnett JA, Nigrovic LE. Pediatric Emergency Care Applied Research Network head injury clinical prediction rules are reliable in practice. Arch Dis Child. 2014 May;99(5):427-31.
Lorton F, Poullaouec C, Legallais E, Simon-Pimmel J, Chêne MA, Leroy H, Roy M, Launay E, Gras-Le Guen C. Validation of the PECARN clinical decision rule for children with minor head trauma: a French multicenter prospective study. Scand J Trauma Resusc Emerg Med. 2016 Aug 4;24:98.
Ide K, Uematsu S, Tetsuhara K, Yoshimura S, Kato T, Kobayashi T. External Validation of the PECARN Head Trauma Prediction Rules in Japan. Acad Emerg Med. 2017 Mar;24(3):308-314.
Babl FE, Borland ML, Phillips N, Kochar A, Dalton S, McCaskill M, Cheek JA, Gilhotra Y, Furyk J, Neutze J, Lyttle MD, Bressan S, Donath S, Molesworth C, Jachno K, Ward B, Williams A, Baylis A, Crowe L, Oakley E, Dalziel SR; Paediatric Research in Emergency Departments International Collaborative (PREDICT). Accuracy of PECARN, CATCH, and CHALICE head injury decision rules in children: a prospective cohort study. Lancet. 2017 Jun 17;389(10087):2393-2402.
Nakhjavan-Shahraki B, Yousefifard M, Hajighanbari MJ, Oraii A, Safari S, Hosseini M. Pediatric Emergency Care Applied Research Network (PECARN) prediction rules in identifying high risk children with mild traumatic brain injury. Eur J Trauma Emerg Surg. 2017 Dec;43(6):755-762.
References (Expert Commentary)
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1-16.
Sharp AL, Huang BZ, Tang T, et al. Implementation of the Canadian CT Head Rule and Its Association With Use of Computed Tomography Among Patients With Head Injury. Ann Emerg Med. 2017;33(0):1505-1514.
Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294(12):1511-1518.
Rosenberg A, Agiro A, Gottlieb M, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. October 2015:1.
Schuur JD, Carney DP, Lyn ET, et al. A top-five list for emergency medicine a pilot project to improve the value of emergency care. JAMA Intern Med. 2014;174(4):509-515.
Mills AM, Raja AS, Marin JR. Optimizing Diagnostic Imaging in the Emergency Department. Acad Emerg Med. 2015:n/a-n/a.
Schriger DL, Elder JW, Cooper RJ. Structured Clinical Decision Aids Are Seldom Compared With Subjective Physician Judgment, and are Seldom Superior. Ann Emerg Med. 2016.
Babl FE, Oakley E, Dalziel SR, et al. Accuracy of Clinician Practice Compared With Three Head Injury Decision Rules in Children: A Prospective Cohort Study. Ann Emerg Med. 2018;71(6):703-710.
Stiell IG, Lesiuk H, Wells GA, et al. The Canadian CT head rule study for patients with minor head injury: Rationale, objectives, and methodology for phase I (derivation). Ann Emerg Med. 2001;38(2):160-169.